ER/UC-OI Index™ Innovative Orthostatic Intolerance & POTS Detection for Faster Diagnosis
- Aug 22, 2025
- 13 min read
Updated: Aug 31, 2025
Introduction
Orthostatic intolerance and POTS are increasingly more common, disabling, and often missed until someone reads the chart as a timeline. The signs of these conditions already lives in routine positions and vitals clinicians collect anyway: triage after upright waiting, first supine on the bed, a short bathroom walk and return, and discharge. POTS is defined by orthostatic symptoms plus a heart rate rise of at least 30 bpm in adults or at least 40 bpm in adolescents within 10 minutes upright, in the absence of orthostatic hypotension. These cutoffs are anchored by the Heart Rhythm Society consensus and recent clinical reviews (Sheldon et al., 2015; Raj et al., 2022).
Accuracy and nuance matter. Pulse oximetry can overestimate oxygen saturation in people with darker skin, which can widen the gap between numbers and symptoms unless the limitation is documented in the note (Sjoding et al., 2020; Fawzy et al., 2022).
Important distinction from PEM. Overexertion in POTS usually causes immediate orthostatic symptoms that improve when recumbent. A delayed multi-day crash is not required for POTS. If delayed crashes occur, that suggests a PEM phenotype on top. This paper centers records-first orthostatic recognition. PEM can be added when the chart shows the delayed pattern (Sheldon et al., 2015; Raj et al., 2022).
Early recognition matters clinically and economically. The longer patients remain unstabilized, the more cycles of orthostatic surges entrench disability and slow recovery. On the system side, a records-first pathway lowers payer spend from duplicate testing and avoidable revisits, and makes trial recruitment cheaper by expanding computable cohorts for EHR screening (Getz, Tufts CSDD; Kalankesh et al., 2024).
What OI and POTS are
POTS
Orthostatic symptoms with a heart rate rise of at least 30 bpm in adults, or at least 40 bpm in adolescents, within 10 minutes upright, without orthostatic hypotension. Symptoms often include lightheadedness, palpitations, tremulousness, generalized weakness, blurred vision, exercise intolerance, and fatigue. Name tremulousness explicitly in the note to prevent psych mislabeling (Sheldon et al., 2015; Raj et al., 2022).
Orthostatic hypotension
A sustained drop of at least 20 mm Hg systolic or at least 10 mm Hg diastolic within 3 minutes upright, with neurogenic and non-neurogenic patterns described in consensus guidance (Freeman et al., 2011).
Heat and cold intolerance are common in dysautonomia. Patients may freeze or shiver while upright in triage lines, then look normal when supine. Document patient report even if not visible later (PoTS UK, 2023; Cleveland Clinic, 2024).
Why this matters now
Underdiagnosis is the norm. Major centers and reviews describe POTS as common and frequently missed in routine care. Authoritative sources place population prevalence around 0.2–1 percent, which implies roughly 1–3 million Americans pre-pandemic, yet many remain undiagnosed in practice (Vernino et al., 2021; Cleveland Clinic, 2024).
Diagnostic delays are long. Large community survey work reported a median diagnostic delay near 24 months with frequent misdiagnosis, often with psychosomatic labels. Recent outcomes work echoes persistent delays reported by patients (Shaw et al., 2019; Boris et al., 2024).
Traditional baseline is already large. Leading institutions commonly cited 1–3 million Americans with POTS before COVID-19, derived from the 0.2–1 percent prevalence range. Post-COVID clinical sources now describe sharp rises in POTS burden relative to those baselines (Johns Hopkins Medicine, 2024; Fedorowski, 2023).
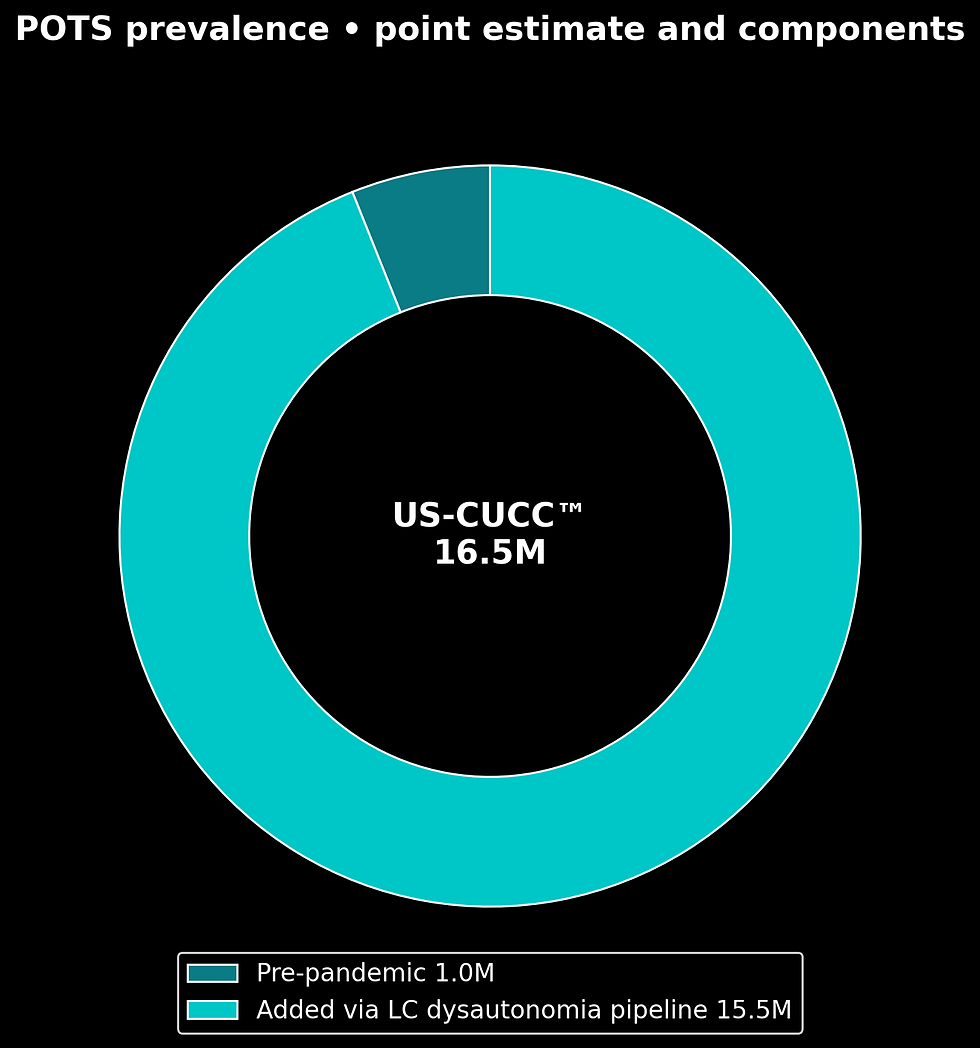
US-CUCC™ (G) POTS Prevalence Model
Start with the pre-pandemic POTS baseline used by clinicians and advocacy groups. Add the Long COVID dysautonomia pipeline with transparent parameters drawn from cardiology and autonomic literature. Publish a point estimate with a range, consistent with CDC burden-estimation practice for under-ascertained conditions (CDC methodology analog; Fedorowski, 2023; Jamal et al., 2022).
Formula. Corrected POTS = Pre-pandemic POTS + (Long COVID × % with dysautonomia × % that map to POTS if formally tested).
Inputs. Pre-pandemic POTS about 1,000,000; Long COVID base 35,000,000; dysautonomia among Long COVID frequently reported at high levels across cohorts, here modeled at 75 percent as an upper-midpoint; of that dysautonomia group, 50–70 percent map to POTS if formally tested in clinic series and academic-center briefs (Fedorowski, 2023; Jamal et al., 2022; Dani et al., 2021).
Steps. New dysautonomia = 35,000,000 × 0.75 = 26,250,000. Corrected POTS = 1,000,000 + (26,250,000 × 0.50 to 0.70).
Conservative scenario 14,125,000.
High scenario 19,375,000.
Public reporting range 14.0–18.0 million.
Point estimate 16.5 million. This mirrors CDC style: disclose assumptions, show the math, publish a point with uncertainty bounds (CDC, burden methods).
The CYNAERA ER/UC-OI Index™
A safer, auditable way to detect OI and POTS from routine records without new provocation.
Step 1. Positions and timing to extract. Pull four phases that often already exist: triage after upright waiting, first supine after bed assignment, post-ambulation vitals such as bathroom walk and return, and discharge vitals before exit. These are standard nursing flowsheet moments in emergency care (AHRQ ESI; ED nursing flowsheet guides).
Step 2. Patient story paired to chartable signals. “I feel dizzy or close to fainting; my heart races when I stand” → apply POTS thresholds when BP does not meet OH cutoffs (Sheldon et al., 2015; Raj et al., 2022).
“I get tremors when I stand” → tremulousness is a recognized POTS symptom, name it to prevent psych mislabeling (Raj et al., 2022; Sandroni et al., 2020).
“I freeze or shiver in the waiting line” → thermoregulatory dysautonomia; note the context even if absent when supine (PoTS UK, 2023; Cleveland Clinic, 2024).
“Breathing feels off but oxygen looks normal” → if darker skin, add the pulse-ox accuracy caveat with citations to NEJM, JAMA IM, and FDA draft (Sjoding et al., 2020; Fawzy et al., 2022; FDA, 2024–2025).
Step 3. Vitals to capture or extract. Heart rate: supine-to-upright increase of at least 30 bpm in adults or at least 40 bpm in adolescents inside 10 minutes is POTS positive if BP does not meet OH thresholds (Sheldon et al., 2015; Raj et al., 2022).
Blood pressure: supine-to-upright drop of at least 20 systolic or at least 10 diastolic within 3 minutes defines OH; note initial OH where captured (Freeman et al., 2011).
SpO₂ caveat: if symptoms suggest dyspnea and SpO₂ reads normal in a darker-skinned patient, document known device bias and rely on the full clinical picture (Sjoding et al., 2020; Fawzy et al., 2022; FDA, 2024–2025).
Temperature context: low-grade chills or heat sensations while upright support a dysautonomia pattern (PoTS UK, 2023; Cleveland Clinic, 2024).
Patient prompt that helps. “Please check heart rate and oxygen before and after I walk to the bathroom, and write how I feel at each point.” These measurements are routine, appear in nursing flowsheets, and are safe in standard practice (AHRQ ESI; ED nursing flowsheets).
Opportunistic OI and POTS testing without NASA Lean
You do not need a formal stand script to capture orthostatic change. Use organic moments the system already records.
Five organic micro-protocols you can score from the chart.
Triage to bed delta, upright to supine. Pair triage after the waiting line with first supine. A large HR drop when recumbent, with an upright–supine delta that meets POTS thresholds, supports diagnosis. Note symptom relief when recumbent (Sheldon et al., 2015).
Bathroom-walk pair. Record HR and BP right before a short walk and on return. A post-ambulation HR spike of about 20 bpm supports OI, and sustained deltas that meet POTS thresholds inside 10 minutes are POTS positive if no OH. Add the pulse-ox caveat when indicated (Raj et al., 2022; Sjoding et al., 2020; Fawzy et al., 2022).
Scale-stand snapshot. Many ERs document vitals while standing on the scale; pair with immediate seated or supine to show excessive tachycardia that resolves in recumbence (ED nursing flowsheets).
Chair-to-stand active stand. If supine is impractical, use a 5 minute seated baseline, then 10 minutes standing with readings at 1, 3, 5, 8, 10 minutes. Seated baselines can underestimate the true delta versus supine. Interpret conservatively (Sheldon et al., 2015; Raj et al., 2022).
Poor man’s tilt from home or bedside devices. When patients bring portal data, look for 5–10 minutes supine followed by 10 minutes standing with serial readings; this maps to accepted stand testing in clinic materials (PoTS UK, 2023).
Fast scoring rubric for the ER/UC-OI Index. Give +1 for each, then interpret together. Upright HR meets POTS delta on at least two standing readings inside 10 minutes. No OH by 3 minutes. Symptoms improve with recumbence in the same encounter. Post-ambulation HR spike of about 20 bpm with symptom reproduction paired to a recorded position sequence. 0–1 normal or indeterminate. 2 borderline OI. 3–4 OI or POTS positive pending clinical confirmation and exclusion of mimics per consensus lists (Sheldon et al., 2015; Freeman et al., 2011).
How to audit past visits for OI and POTS
Clinician workflow in the EHR
Pull a 90-day timeline of ER, urgent-care, and primary-care encounters. Review triage after upright waiting, bed vitals for supine, any post-ambulation vitals such as bathroom trips, and discharge vitals for recovery pattern. Compute HR and BP deltas and apply POTS and OH thresholds. Write one integrated note that links timing, symptoms, vitals, and negative acute workups. Avoid duplicative tests when the chart already contains what you need (Sheldon et al., 2015; Freeman et al., 2011; Raj et al., 2022).
Patient workflow in the portal
Download after-visit summaries. Mark dates that fall inside 72 hours if there are clusters. Highlight triage, bed, walking, and discharge vitals. Note dizziness, palpitations, visual blurring, chest pressure, brain fog, tremor, heat or cold intolerance, and any freeze or shiver episodes. Ask your clinician to apply consensus thresholds to your timeline and document the result (PoTS UK, 2023; Cleveland Clinic, 2024).
Diagnosis lag and mislabeling
Large community data show a median of about 24 months to diagnosis, with frequent misdiagnosis, often with psych labels. Records-first recognition targets that gap by using data that already match consensus thresholds (Shaw et al., 2019; Boris et al., 2024).
Economic impact in the United States
In a large international cohort, about 70 percent reported income loss due to POTS in the prior year, with a substantial share losing more than 10,000 dollars in a single year, and meaningful out-of-pocket costs (Bourne et al., 2021). Multiply 1–3 million pre-pandemic by even 10,000 dollars median income loss and you see 10–30 billion dollars per year in household losses. At the corrected point estimate of about 16.5 million, the illustrative household impact approaches 165–297 billion dollars per year before medical costs. Earlier records-first diagnosis moves that curve by shortening delay and stabilizing sooner (Bourne et al., 2021).

Payer partnership and incentive design
Why payers care
Faster recognition from records cuts avoidable spend in three places: duplicate testing triggered by diagnostic uncertainty, ED and urgent-care revisits during undiagnosed flares, and work disability with caregiver time that spills into claims. This matches mainstream guidance to evaluate orthostatic patterns safely and document without unnecessary provocation (Sheldon et al., 2015; Raj et al., 2022).
Pilot mechanics and contract options
Target members with repeat ER or urgent-care use, post-viral flags, or autonomic clusters. Use a CYNAERA auto-scan packet plus one 20–30 minute tele evaluation using the ER/UC-OI Index. Outcomes include time to diagnosis, duplicate test rate, revisits, days to accommodation, and member-reported function. Contracts can use shared-savings or fixed-fee plus KPI bonuses, copay support, and de-identified claims for pre-ranking with return of audit packets and confirmations.
Scale-up through short remote training
A one-hour remote training that teaches the ER/UC-OI Index workflow, orthostatic capture, and safe documentation can be delivered to as many clinicians per site as desired. Because POTS is visible on vitals, throughput can exceed PEM confirmation rates (NHS England, 2024; Raj et al., 2022).
Capacity math
Conservative cadence at 5 confirmations per clinician-week yields about 260,000 confirmations per 1,000 clinicians per year. With pre-ranked charts and records-first cadence at 10–12 focused evaluations per clinician-day, output is about 1,610–1,932 confirmations per clinician-year on a 230-day schedule. At 5,000 clinicians that is roughly 8.0–9.7 million confirmations per year. Because OI and POTS are visible on vitals, scale is high (Sheldon et al., 2015; Raj et al., 2022).
CYNAERA automation modules
CYNAERA’s OI and POTS bundle reads what is already in the record, builds the timeline, and drafts the note so visits stay inside standard slots. Pre-Triage assembles triage and nursing flowsheet vitals into a clinician-ready brief, while SymCas and SymCas-Timeline generate the encounter timeline that anchors clustering and trend review. CrashSync aligns any labs to the same window so inflammation or electrolyte context is visible without new draws. At the orthostatic layer, EPTS flags dysautonomia patterns at ER intake and PDOW mines sit-to-stand sequences for POTS thresholds. If the chart is noisy, Diagnostic Clustering Suppression Flag and Late-Day Flare Pattern Flag correct common grouping misses and capture evening spikes that often appear in real-world workloads.
VitalGuard can add environmental context when available, and Electrolyte Stability Index surfaces simple correction opportunities that help stabilize before formal autonomic referral. ICDx-USA provides coding suggestions that match the narrative so the confirmation and plan are billable and searchable. For pediatrics, POTS_MCAS_Pediatric_Referral computes urgency and routing in one click. For throughput and follow-through, Telehealth Access Optimizer routes cases to clinicians with open remote capacity, MindMirror with the Cognitive Fluctuation Index captures co-occurring cognitive changes that often travel with orthostatic flares, and EDRS/EDRS+ tracks short-term revisit risk after discharge so programs can show fewer bouncebacks. The output is a ready-to-sign note and a one-page patient summary for the portal.
Global implementation and impact
United Kingdom: remote consultations are standard practice across NHS pathways, with national guidance describing complete care episodes by phone or video (NHS England, 2024).
South Korea: national policy now allows telemedicine broadly across hospitals and clinics and internet access is near universal, which supports portal pulls at scale (Gov. of Korea, 2024–2025 briefs).
Brazil: Law 14.510/2022 permanently authorizes telehealth nationwide, and household internet access is in the 90 percent range, supporting broad outreach for records-first orthostatic review (Brazil, Law 14.510/2022; IBGE, 2024).
Two-year diagnosis lift with short remote training
One-hour remote training, unlimited seats per site. With 10–12 targeted evaluations per clinician-day and typical confirmation rates in pre-ranked lists, output is about 1,800–2,200 confirmations per clinician-year. If each country onboards 100 clinicians, two-year output is roughly 720,000–880,000 confirmations without tilt tables, using accepted thresholds (Sheldon et al., 2015; Raj et al., 2022; NHS England, 2024).

Clinical-trial acceleration effect
When a larger share of the POTS population is already diagnosed and documented from routine positions and vitals, sponsors recruit from a pre-confirmed pool instead of cold screening. That raises the eligibility hit rate, shortens time to target N, and lowers cost per randomized participant. This is consistent with evidence that EHR phenotyping and computable criteria improve recruitment efficiency across indications (O’Brien et al., 2021; McCord et al., 2019; Kalankesh et al., 2024).
Simple example. Need 600 participants. With cold outreach you might find about 1 in 20 eligible so roughly 12,000 contacts. With a large diagnosed pool and records-first confirmation you may reach 1 in 4 so about 2,400 contacts. Screening spend and timeline fall because fewer people are contacted to reach the same N. Published experience with EHR pre-screens mirrors this direction of effect (O’Brien et al., 2021; Kalankesh et al., 2024).
Optional add-on: opportunistic PEM detection
If the chart shows clustered encounters inside 72 hours and the narrative fits a delayed crash, apply the CYNAERA ER/UC-PEM Index in the same record review to document PEM safely. That creates a unified dysautonomia plus PEM note that is computable for trials and coding.
Conclusion
The CYNAERA ER/UC-OI Index turns routine clinical data into a high-throughput diagnostic engine for orthostatic intolerance and POTS. No tilt table required. Using positions and vitals already in the chart, clinicians can confirm OI and POTS safely, document it in a computable way, and move immediately to stabilization and follow-up. With one hour of remote training and unlimited seats per site, thousands of clinicians can confirm hundreds of thousands to more than a million cases per year in telehealth. That shortens diagnostic lag, reduces repeated workups, expands pre-confirmed cohorts for trials, and addresses a macro burden measured in the tens to hundreds of billions of dollars in household income losses that can be reduced by records-first recognition (Bourne et al., 2021; Sheldon et al., 2015; Raj et al., 2022).
References
Agency for Healthcare Research and Quality (AHRQ). Emergency Severity Index (ESI) triage handbook and guidance on vital signs in triage.
Boris JR, et al. Long-term outcomes and labeling in postural orthostatic tachycardia syndrome. Journal of the American Heart Association (2024).
Bourne KM, et al. Economic burden and employment impact of postural orthostatic tachycardia syndrome. Journal of Internal Medicine (2021).
Bryarly M, et al. Postural orthostatic tachycardia syndrome: pathophysiology, diagnosis, and management. Journal of the American College of Cardiology (2019).
Centers for Disease Control and Prevention (CDC). Burden-estimation methodology for under-ascertained conditions.
Cleveland Clinic. Dysautonomia and POTS clinical overviews (2024).
Dani M, et al. Autonomic dysfunction in Long COVID. Clinical Medicine (2021).
U.S. Food and Drug Administration (FDA). Pulse oximeter performance across skin tones, draft communications (2024–2025).
Fawzy A, et al. Pulse oximetry accuracy and skin tone. JAMA Internal Medicine (2022).
Fedorowski A. Post-acute SARS-CoV-2 and autonomic dysfunction. Nature Reviews Cardiology (2023).
Freeman R, et al. Consensus definition of orthostatic hypotension. Clinical Autonomic Research (2011).
Instituto Brasileiro de Geografia e Estatística (IBGE). Household internet access indicators, Brazil (2024).
Jamal SM, et al. Autonomic dysfunction after COVID-19. Journal of the American College of Cardiology (2022).
Johns Hopkins Medicine. POTS overview and prevalence framing (2024).
Kalankesh LR, et al. Electronic health records and computable phenotypes in clinical trials, systematic review. 2024.
McCord KA, et al. Using electronic health records in randomized trials. Trials (2019).
NHS England. National guidance for remote consultations (2024).
O’Brien EC, et al. EHR-based identification for trial screening in cardiovascular research. 2021.
PoTS UK. Patient resources on temperature regulation and stand tests (2023).
Raj SR, Fedorowski A, Sheldon RS. Diagnosis and management of POTS. CMAJ (2022).
Sandroni P, et al. Autonomic disorders and tremulousness, clinical context notes. 2020.
Shaw BH, et al. Diagnostic delay and misdiagnosis patterns in POTS. Journal of Internal Medicine (2019).
Sheldon RS, et al. Heart Rhythm Society expert consensus statement on POTS. Heart Rhythm (2015).
Sjoding MW, et al. Racial bias in pulse oximetry measurement. New England Journal of Medicine (2020).
Government of the Republic of Korea. National telemedicine expansion communications (2024–2025).
Vernino S, et al. Autonomic disorders: state-of-the-science report. Autonomic Neuroscience (2021).
Federative Republic of Brazil. Law 14.510/2022, permanent national telehealth authorization.
Author’s Note:
All insights, frameworks, and recommendations in this white paper reflect the author's independent analysis and synthesis. References to researchers, clinicians, and advocacy organizations acknowledge their contributions to the field but do not imply endorsement of the specific frameworks, conclusions, or policy models proposed herein. This information is not medical guidance.
Applied Infrastructure Models Supporting This Analysis
Several standardized diagnostic and forecasting models developed through CYNAERA were utilized or referenced in the construction of this white paper. These tools support real-time surveillance, economic forecasting, and symptom stabilization planning for infection-associated chronic conditions (IACCs).
Note: These models were developed to bridge critical infrastructure gaps in early diagnosis, stabilization tracking, and economic impact modeling. Select academic and public health partnerships may access these modules under non-commercial terms to accelerate independent research and system modernization efforts.
Licensing and Customization
Enterprise, institutional, and EHR/API integrations are available through CYNAERA Market for organizations seeking to license, customize, or scale CYNAERA's predictive systems.
Learn More: https://www.cynaera.com/systems
About the Author
Cynthia Adinig is an internationally recognized systems strategist, health policy advisor, and the founder of CYNAERA, an AI-powered intelligence platform advancing diagnostic reform, clinical trial simulation, and real-world modeling for infection-associated chronic conditions (IACCs). She has developed 400+ Core AI Frameworks, 1 Billion + Dynamic AI Modules. including the IACC Progression Continuum™, US-CCUC™, and RAEMI™, which reveal hidden prevalence, map disease pathways, and close gaps in access to early diagnosis and treatment.
Her clinical trial simulator, powered by over 675 million synthesized individual profiles, offers unmatched modeling of intervention outcomes for researchers and clinicians.
Cynthia has served as a trusted advisor to the U.S. Department of Health and Human Services, collaborated with experts at Yale and Mount Sinai, and influenced multiple pieces of federal legislation related to Long COVID and chronic illness.
She has been featured in TIME, Bloomberg, USA Today, and other leading publications. Through CYNAERA, she develops modular AI platforms that operate across 32+ sectors and 180+ countries, with a local commitment to resilience in the Northern Virginia and Washington, D.C. region.




Comments